Minimizing the use of natural resources is one of today’s biggest concerns, and in this view, recycling procedures are essential for the sustainability of our existing way of life.
Reusing or recycling used lubricating oil has two benefits: It gets rid of a potentially dangerous residue and lessens the need to extract more petroleum to make basic lubricating oils.
Re-refining methods, like other chemical processes, were the focus of extensive study aimed at process improvement in the sense of cost reduction and primarily the environmental impact.
Lubricating oils are not consumed during use, unlike other petroleum derivatives like diesel, gasoline, and kerosene. But, over the course of their lifespan, lubricating oils become contaminated with a variety of impurities, such as worn metallic particles from engine cylinders, deterioration of additives, unburned fuel, gases produced by combustion, dirt, etc., and lose their chemical and physical quality and their ability to effectively lubricate.
Base oil is a petroleum product made by fractionally distilling crude oil. It is the primary raw material used to make lubricants including hydraulic oil, gear oil, engine oil, and others.
The contaminants are then removed from this old oil during the refining process to produce new base oil. By adding the necessary additives, this previously refined base oil can be utilized to create new lubricants.

Benefits of used oil- Re-Refining/Recycling
- Since our supply of crude oil is finite, recycling spent oil allows us to conserve non-renewable energy sources for future generations.
- Used oil is inherently toxic. We can protect the environment by recycling used oil because even a small bit of it can kill many people and pollute enormous quantities of water on Earth.
- The used oil recycling industry is lucrative, and thanks to it, consumers may get recycled base oil for less money than they would pay for virgin base oil.
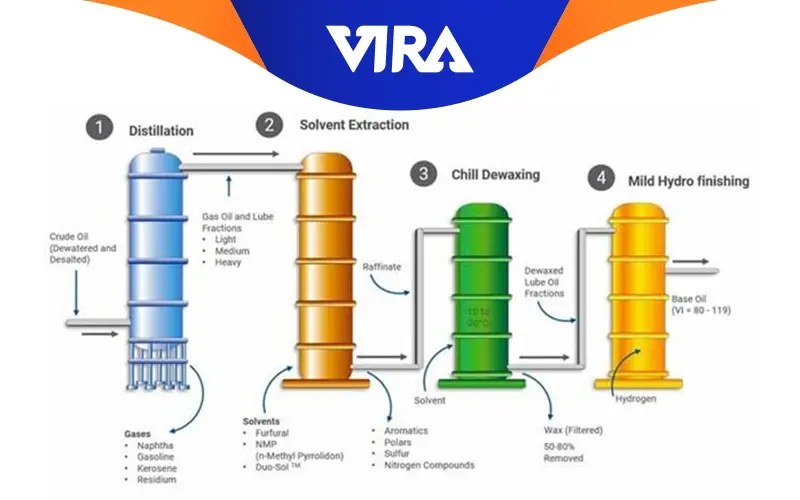
Re-refining phases of used base oil
Because there haven’t been any significant modifications to the base oil’s components, recycling used oils can be considered.
All currently used procedures go through the same re-refining phase. They are as follows:
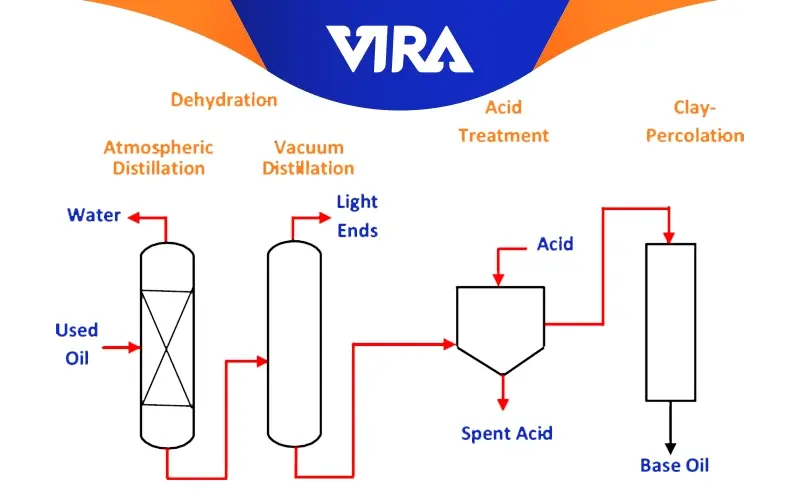
1. Dehydration:
Sedimentation and dehydration processes are used to separate the water and solid impurities in the oil. Dehydration is the first step in recycling used oil. Used oil is moved from the storage tank to the dehydration reactor, where it is heated to a specific temperature to remove water and fuel (Which is also known as light ends). To reduce surface tension, the procedure is carried out under a vacuum of 600–700 mmHg. As the temperature rises, the fuel and water evaporate first and then pass through a condenser to return to liquid form, where they are collected in receivers.
2. Distillation:
Following the dehydration procedure, the used oil is transferred to an evaporator for distillation where it is heated from 320 °C to 350 °C under >759.5 mmHg vacuum. As the temperature rises, the base begins to evaporate from the used oil and passes through condensers, returning it to liquid form where it is collected in receivers. Asphalt sometimes referred to as the bottom, residue, bitumen, etc., is left behind at the bottom of the evaporator after all of the base oil has evaporated and is also collected in the receiver.
1. atmospheric distillation
The atmospheric distillation technique is typically used to separate oil’s volatile components, fuel leftovers (such as gasoline and diesel), solvents, and lubricant components with low boiling points. Light hydrocarbons that have been separated can be used to provide energy.
2. Vacuum Distillation :
Vacuum distillation is used to isolate some heavy fractions that would not distill at atmospheric pressure without being harmed during distillation. It is applied to determine the flash point and starting base oil viscosity properties. The four different viscosity fractions (or distillates) that are produced as a result of this process are used to create the finished oil products.
3. Refining or bleaching :
Refining or bleaching is performed to remove unwanted chemical structures (rings, etc.) from the base oil to reduce the tendency of the base oil to age in service and The density, viscosity index, lifespan, and color enhancement of old oils are all improved by removing these materials. Acid filtering, solvent (propane) extraction, vacuum distillation, and partial hydrogenation are used to separate additives and by-products of aging (such as sludge, etc.). Using the treating process, any leftover material (additives, aging by-products, and refining reaction products) that could not be removed using the techniques used in the previous phases is finally separated. The treatment process is typically carried out by hydrotreating, absorbents like colored clays, or extraction by a different solvent (such as furfural). When it comes to removing dangerous substances (nitrogenous, oxidized, sulfurous compounds, and metals) from oil, hydrotreating is a procedure with low-performance requirements. The separation process for used oil re-refining ends with this stage. In order to more fully understand why base oils exhibit different qualities, it is necessary to briefly describe the various refining processes listed below.
- Sulfuric Acid Method
- Bentonite clay method
- Solvent Extraction
- Catalytic Hydrogenation
4. De-asphalting
The de-asphalting procedure is a stage in which the heavy asphalt residue is taken out of the beneficial distillate fractions.
5. De-waxing
De-waxing is a process that lowers the wax content of the base oil to enhance the oil’s low-temperature qualities.
6. Blending
The final step in creating a finished lubricating oil is blending. It entails combining several base oils to achieve the required viscosity and adding certain additives to make sure the resulting oil has the right qualities to deliver the desired lubricating ability.

used base oil refining process (bleaching process)
There are many techniques around the world to recycle used oil, including solvent extraction, distillation, Sulphuric acid/clay refining, and clay. The methods listed below are used to recycle used oil.
- Refining with an acid called Sulphuric acid method
- Refining using color adsorbent clay (Bentonite clay method)
- Solvent extraction
- Catalytic Hydrogenation (hydrotreating)
Acid Technique
The three steps of this procedure are as follows.
- primary distillation; heat to a temperature of around 360 ± 20 °C
- Acid wash; eliminating the acid sludge two days after freezing the oil and combining it with roughly 8% sulfuric acid by weight.
- Secondary distillation: The procedure involves heating the oil to about 320 degrees Celsius, mixing in about 12% color remover, bringing the temperature down, and then transferring it to the filter press. This method is no longer used because of significant environmental resistance, high energy consumption, three stages, and upfront outlay. It also required three different types of infrastructure, and its most important issue was acid sludge. Burnt oil is refined using this approach over around four days.
The oxidation components in the oil are polar and penetrate the polar bentonite soil, where they separate from the oil. These oxidized components in the oil cause the oil to become more colored.
Shear stability is improved in the acid technique by eliminating the polymer, ash is decreased by removing the metal, and the viscosity index and oxidation stability are improved by deleting the aromatic compounds.
Clay Technique
The acid-clay method, also known as the modified Meiken process, was the first industrial technique created to recover used lubricating oil.
The clay method only incorporates portion C of the acid approach and is a one-step process. With this technique, the wasted oil is added to the cooking pot simultaneously as a cheap dye-adsorbent addition. The light components are separated at the final manufacturing stage only after single heating. The remaining material, oil, and clay that can absorb color are then transferred to the filter press. The base oil is obtained once the clay has been separated.
This technology requires less initial expenditure than the acid and distillation process. This technology is replacing the acid approach. However, the cost of operation is relatively high because sulfuric acid and clay are required in such large quantities. The generation of acid sludge, a hazardous byproduct with challenging treatment, is the primary drawback of the acid-clay process.
Its key advantage is that it is single-stage, with lower energy, labor, and initial investment costs than the acid method. It also has no acid sludge and can compete with the acid method in quantitative and qualitative terms. The inexpensive dye-absorbing clay used in this procedure is 23% ± 2%.
With this technique, the complete recycling process takes less than a day.
One disadvantage of the acid-clay process is that it can only create group I base oils, which restricts the consumer market and has a significant negative impact on profitability in comparison to alternative technologies.
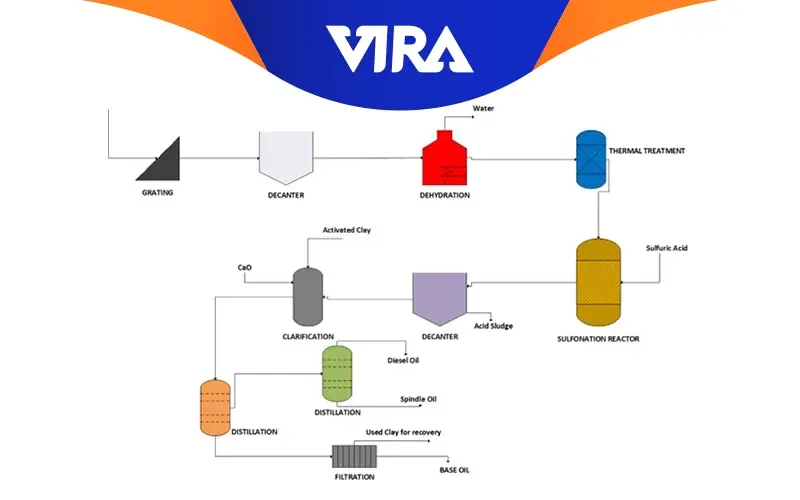
Basic Process Flow Diagram for Acid-Clay Process
- The used oil is first passed through grids to remove coarse particulates.
- then it is put through a decanter to help separate any free water that may be present in the oil.
- The feed is then dehydrated in order to remove any remaining water that was still maintaining an equilibrium with the oil
- In order to stimulate the breakdown of the additives used in the lubricating oil formulation and ensure that no chemical degradation occurred during the lubricating life cycle, the used oil is afterwards subjected to a heat treatment at temperatures around 340 oC.
- The thermally and chemically damaged products are separated from the base oil in the subsequent phase, which involves directing the spent lubricating oil to a reactor and adding sulfuric acid.
- The oil is transferred to a new decanter where base oil, which is in a liquid phase, and acid sludge, which is in a solid phase and contains the degradation products during the lubricating life cycle, are separated.
- To eliminate chemicals that could give the base oil color, activated clay is added to the oil during the clarifying process. By adding calcium oxide (CaO), the base oil’s acidity is adjusted in this step.
- The oil next goes through distillation procedures to get rid of the lighter chemicals that might have gotten into the old lubricating oil throughout its lifetime or were created by thermal cracking during the process.
- Base oil filtration—the last stage of the process—is used to remove the clay from the base lubricating oil before it is pumped to storage tanks for later use.
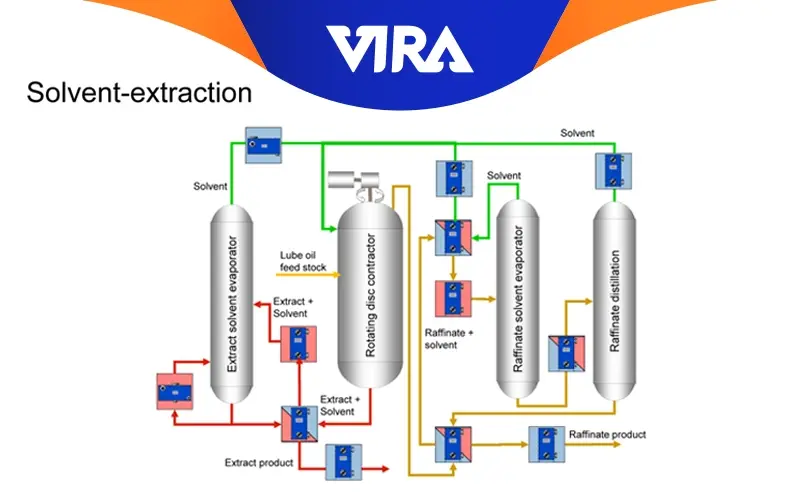
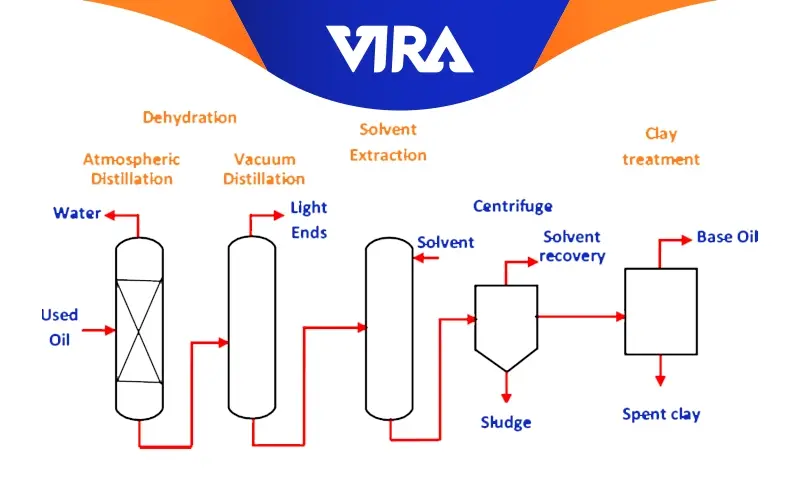
Solvent Extraction
The term “solvent extraction” refers to the process of liquid extraction used to remove the majority of the ring structures and aromatics (weak, unwanted components) from oil distillates. Solvents like phenol, furfural, and sulfur dioxide are typical and appropriate. As an extractant for the refinement of paraffinic oils, furfural is widely utilized. Base stocks that are neutral oils are produced as a result, and an extract fluid with a high aromatic content is produced. These are utilized to make process oils and fuel oils.
The components that are left over after solvent extraction are dewaxed to increase low-temperature fluidity and occasionally hydro-finished with hydrogen gas to enhance color and stability even more.
The degree to which temperatures and pressures are applied during the hydro-finishing process affects the base oil’s final quality. The base oils are now prepared for careful blending with the right additions to give the finished oil the desired physical, chemical, and performance characteristics.
Catalytic Hydrogenation (hydrotreating)
The refining procedure known as catalytic hydrogenation (also known as hydrotreating) involves subjecting the crude distillates to a chemical reaction with hydrogen while a catalyst is present, at temperatures as high as 420 degrees C (800 degrees F) and pressures as high as 3,000 psi. Several base oil refiners and lubricant producers choose hydrotreating techniques because of the improved quality of the base oils and the negligible amount of waste hydrocarbons produced.
At the refinery, hydrocarbon structures that were formerly considered waste streams are now transformed into useful base oils. More than 90% of the aromatic content is transformed into more valuable hydrocarbons during hydrotreating.
There are three different kinds of hydrotreating:
- Hydrofinishing: Hydrofinishing” is a moderate technique that removes the residual remnants of unwanted chemicals.
- Hydrotreating: “Hydrotreating” is a refinement process that uses hydrogenation to enhance the distillates’ color, aroma, oxidation stability, and demulsifying qualities.
- High-pressure hydrogenation: The process of “high-pressure hydrogenation,” also known as “hydrocracking,” allows for the total elimination of undesirable substances and almost complete conversion of the aromatic and naphthenic rings into branched paraffin.

